EXTRACT OF THE PAPER
Biological phosphorus removal BY Pure CULTURE
of lampropedia spp.
Stante L., Cellamare C. M., Malaspina F., Bortone
G. and Tilche A.
ENEA - Sezione Depurazione e Ciclo dell’Acqua -
Via Martiri di Monte Sole, 4
I-40129 Bologna - Italy
Abstract
Lampropedia spp. is a
gram-negative, Neisser-positive coccus that was isolated from EBPR (enhanced
biological phosphate removal) activated sludge laboratory plants operating on
dairy and piggery wastewaters. In aerobic growth tests carried out on sodium
acetate, Lampropedia spp. stored PHB
up to 12% w/w. Biomass yield was estimated 0.55 gVSS·gHAc-1, and
specific growth rate 0.045 h-1. The experimental maximum acetic acid removal rate resulted 71.86
mgHAc·gVSS-1·h-1 with a semisaturation constant of 71.78 mg·L-1. Batch tests were carried out to check
whether Lampropedia spp. was capable
of enhanced biological phosphorus removal. Under anaerobic conditions, Lampropedia spp. sequestered acetate and
stored PHB with an average conversion factor of 0.33 mgPHB·mgHAc-1. The
measured maximum PHB storage capacity was 31% w/w, with a maximum specific PHB
accumulation rate of 17 mgPHB·gVSS-1·h-1 and a
specific anaerobic acetate uptake rate of 57 mgHAc·gVSS-1·h-1. The
experimental ratio between phosphorus released and acetate uptaken was low, in
average 0.044 mgPO4-P·mgHAc-1, with a specific rate ranging from 1.7 to 3.6 mgPO4-P·gVSS-1·h-1 at pH 7.5.
Despite of the low figure, fractionation analyses showed that in anaerobic
conditions the released phosphate comes from cell polyphosphate degradation.
Therefore, the whole results allow to conclude that Lampropedia spp. can be classified amongst the phosphorus
accumulating bacteria.
Keywords
Lampropedia spp.,
poly-ß-hydroxibutyrate, enhanced biological phosphorus removal, nutrient removal,
polyphosphate accumulating microorganisms.
Nomenclature
ADM = Chemical Defined Medium
COD = Chemical Oxigen Demand
EBPR = Enhanced Biological Phosphorus
Removal
FID = Flame Ionization Detector
g = Gravity acceleration [L·T-2]
GC = Gas Chromatography
GTA = Green Top Agar
HAc = Acetic Acid
HPIC = High Performance Ion Chromatography
mod. = Modified
PHA = Polyhydroxialcanoate
PHB = Poly-ß-hydroxibutyrate
PHV = Polyhydroxivalerate
poly-P = Polyphosphates
rpm = Rounds per minute
rs = Specific Substrate Removal Rate [T-1]
rsac = Specific Acetic Acid Removal Rate
[T-1]
TSS = Total Suspended Solids
VSS = Volatile Suspended Solids
µ = Specific Growth Rate [T-1]
µobs = Specific Observed Growth Rate [T-1]
Introduction
In the last
years, many studies on pure cultures of activated sludge polyphosphate
accumulating microorganisms (PAO) have been carried out.
The common
characteristics of this heterogeneous group of bacteria are their capability of
synthesizing polyphosphates (poly-P) and polyhydroxyalkanoates as internal
storage compounds; polyphosphates are formed during the aerobic phase, while
PHA are stored during the anaerobic phase (Comeau et al, 1986; Wentzel et al.,
1986; Mino et al., 1987; Mino et al., 1994).
Many different
kinds of poly-P accumulating bacteria have been isolated from activated sludge.
Brodisch and Joyner (1983) found that in several EBPR plants phosphorus removal
was mainly carried out by bacteria belonging to the genera Aeromonas and Pseudomonas,
representing up to 50% of the microbial population. The presence of those two
genera has been confirmed in other studies by Florentz and Hartemann (1984) and
Lötter and Murphy (1985).
Activated sludge
bacteria, belonging to the genus Acinetobacter,
have been isolated for the first time by Fuhs and Chen (1975), who demonstrated
in batch tests that Acinetobacter was
able to take up phosphate and to degrade poly-ß-hydroxibutyrate (PHB) under
aerobic conditions, releasing soluble ortophosphate in anaerobic conditions. On
the other hand, they did not give any figure on acetate uptake in anaerobic
conditions.
Other tests have
been carried out by Deinema et al.
(1980) and Deinema et. al. (1985); in
their experience, Acinetobacter
cultures released soluble ortophosphate without synthesizing PHB under
anaerobic conditions, using acetate, lactate and ethanol as carbon source.
Tandoi et al. (1987) revealed incomplete COD uptake and phosphorus release with
pure cultures of Acinetobacter lwoffi, maintained in anaerobic/aerobic
alternate conditions.
Heymann et al. (1989) emphasised the distinction
between aerobic or microaerophilic growth. In the last condition, Acinetobacter lwoffi was rich either of PHB and poly-P, while
the aerobic cultures had a lower poly-P content.
Bayly et al. (1990) observed phosphorus release without acetate uptake in an Acinetobacter culture that, in spite of
this behaviour, showed enhanced poly-P storage capacity.
Some tests with Acinetobacter calcoaceticus failed. A significant anaerobic acetate uptake was observed without any correlation to phosphate release (Ohtake et al, 1985).
However, it is
now clear that in full scale EBPR plants, many different bacterial groups are
responsible for enhanced biological phosphate removal (Wagner et al., 1994; Bond et al., 1995).
Nakamura et al. (1991) worked in microaerophilic conditions with pure cultures of a
bacteria thought to belong to the genus Micrococcus
and then assigned to a new genus and species Microlunatus phosphorovorus (Nakamura et al., 1995). These bacteria were able phosphate uptake in oxidative
conditions without organic substrate in the medium, and released phosphate when
fed with glucose in anaerobic condition.
More recently,
Ubukata and Takii (1994) hypotised that the enzymatic system for the assumption
of phosphate could be inducted by at least two anaerobic/aerobic cycles. In
that particular case, phosphorus release has been observed with a concomitant
uptake of casamino acid after induction of the cell culture.
The present
study regards a gram-negative coccus, Neisser-positive in aerobic conditions
and Neisser negative in anaerobic conditions, that was observed from EBPR
activated sludge laboratory plants operating on dairy and piggery wastewaters;
it was supposed to be able of enhanced biological phosphate removal. The
microorganism was morphologically identified as belonging to the genus Lampropedia.
The striking
quality of these bacteria is undoubtedly the large cell sheets they use to
build both in solid and liquid medium, a property which gives to Lampropedia the rare prerogative of
being immediately recognized under the microscope (Murray, 1984).
In this paper,
isolation procedures, growth culture conditions and alternate anaerobic/oxic
batch tests for evaluating the enhanced biological phosphorus removal capacity
of Lampropedia spp. are described.
Materials
and methods
Bacteria
Bacteria were
isolated from the activated sludge of a sequencing batch reactor (SBR) treating
dairy and piggery wastewater as described in the isolation procedure.
The single cells
have a rounded, almost cubical shape, and they are arranged in square tablets
of 16-64 or more individual cells. They divide synchronously in a sheet and
alternately in two planes.
Staining and microscopic observation
Gram and Sudan
Black B staining were carried out as reported by Jenkins et. al. (1986); methachromatic granules were stained both with
Pergola method (Pasquinelli, 1981), Albert method (Tandoi et
al., 1990) and Neisser method (Jenkins et
al., 1986); Blue Nile A staining was done as reported by Ostle and Hot
(1982).
Light
microscopic observations were carried out using a Jenalumar A/D Contrast light microscope
(1000x magnification) in bright field, phase contrast, Nomarski interferential
contrast and bright field coupled to fluorescence episcopy; scanning electron
microscope (SEM) observations were carried out using a Philips XL 20.
Media
Two kinds of
media were used for the tests: a modified Green Top Agar (mod.GTA) and a
chemical defined medium (ADM). All media were sterilised by autoclaving at
121°C for 15 minutes.
Modified Green
Top Agar (based on Green Top Agar by Pringsheim (1980) was made with: 2 g of
yeast extract, 1 g of peptone, 1 g of sodium acetate, 100 mL of filtered and
sterilized effluent from dairy wastewater treatment plant, 15 g of agar (only 2
g for semisolid medium), distilled water to 1 L volume and pH adjusted to 7.2.
This medium was used as solid substrate during isolation and maintenance on
Petri plates (100 mm) and tubes (16x200 mm).
The modified
Green Top Agar without agar was used for growth, PHB storage and phosphorus
uptake tests.
ADM was composed
by 160 mg of NH4Cl, 64 mg of KH2PO4, 2 ml of a microelement stock solution (Puttlitz and Seeley, 1968),
and distilled water to 1 L volume. After sterilisation, 1 mg of biotine, 1 µg
of tiamine and a variable amount of sodium acetate (from 500 to 10000 mg,
depending on test conditions) were added after filtration on 0.22 µm sterile
filters. This medium was also used for growth tests. Sodium acetate in data
tests was expressed as acetic acid.
The ADM medium
without KH2PO4 was used in phosphorus releasing tests.
Instruments and analytical methods
Analyses of TSS
and VSS were performed on the residue retained by GF filter following Standard
Methods (1989). The different forms of phosphorus were measured according to
the same manual. Phosphorus fractionation was carried out as reported by De
Haas (1989). Nitrate, ortophosphate and ammonia were determined using a HPIC
(Dionex 4000i). Acetic acid was determined gaschromatographically, using a DANI
8510 GC equipped with a 25 m 0.53 mm 1.2mm capillary wide bore
column (Alltech SO FA bound FSOT) and a FID detector; hydrogen was used as
carrier gas and 2,2-dimethylbutyric acid as internal standard. GC working
conditions were the following: oven temperature from 107°C to 140°C with an
increasing rate of 6 °C·min-1, injector temperature of 220°C and FID
port temperature of 230°C.
PHB was
determined by GC with a modified method as proposed by Braunegg et al. (1978) and Gerhardt et al. (1994). 10 mL of culture media
were centrifuged at 4000 g x 15 min at 4°C. After centrifugation, the
supernatant was discharged and replaced with an equal volume of a solution of sodium
hypochlorite; after incubation at 37°C for 1 hour, the sample was centrifuged
at 9000 g x 15 min at 4°C. The solid fraction was washed with water and
centrifuged at 9000 g x 15 min at 4°C. The solid fraction was then mixed with 2
ml of acidified methanol (3% H2SO4) and 1 ml of
chloroform and then heated for 3.5 h at 100°C.
After the
digestion/methylation, 1 ml of water and 25 µL of internal standard (4000 mg·L-1 2,2-dimethylbutyric
acid solution) were added to the sample; after 10 min. of vigorous shaking,
phase separation follows. 0.1 µL of the chloroform fraction is injected in the
GC, equipped with the same column and detector described above; hydrogen was
used as carrier gas. GC working condition were: oven temperature from 60°C to
120°C with an increasing rate of 4.7 °C·min-1, injector temperature
of 220°C and FID port temperature of 230°C. Pure PHB (Sigma) was used for GC
calibration.
Test methods
The growth tests
were carried out under aerobic conditions in a 2 L well mixed glass bottle
(magnetic stirrer at 60 rpm) with 0.5 L of media (modified GTA or ADM) covered
with a cotton lid. The inoculum was taken from a stock plate colony.
Temperature was kept at 25°C and the initial pH was 7.5.
Cells for
phosphorus release tests were cultured batchwise under aerobic condition in
modified GTA medium.
Phosphorus
release and PHB storage tests were carried out on centrifuged cells (4000 g x
15 min.) washed with phosphorus free media. The washed cells were resuspended
in 0.3-0.5 L of ADM without phosphate in a 2 L glass bottle. Anaerobic
conditions were obtained by bubbling sterilised (by filtration on 0.22 µm
sterile filter) nitrogen gas.
Temperature was
kept at 20°C and, during the tests, pH reached 8.3-8.5.
Results
and discussion
Isolation and identification
After Neisser
staining, these bacteria showed the presence of metachromatic granules; besides,
they were Sudan Black positive (Murray, 1963) and the isolate was also positive
to Albert staining (Tandoi et al,
1990) that, together with Neisser staining, is recommended for evidencing the
presence of volutine granules (Jenkins et
al, 1986). On the basis of these characteristics, it was supposed that Lampropedia might belong to the
functional group of poly-P accumulating microorganisms.
Activated sludge
was sampled from two SBR reactors operating on dairy and piggery wasterwater
and observed by a phase contrast light microscope. Some particular shaped
bacteria belonging to the genus Lampropedia
were observed. Other authors before (Standridge, 1981) have identified Lampropedia spp. in an activated
sludge-trickling filter plant treating cheese factory wastes.
Lampropedia. hyalina was isolated for the first time by
Schroeter (1886) and more in detail by Pringsheim (1955). Lampropedia cells are enclosed in a Gram-negative type of cell
wall; no flagella occur, they are strictly aerobic, chemoorganotrophic and
usually include PHB granules, as described in Bergey’s manual. Lampropedia was first observed in muddy
water and probably its name has been originated because they appear like
tablets “glistening”. They have been isolated in organic matter rich
environment, although a definite ecological niche is not clearly definable.
Growth occurs as
a thin, hydrophobic and extended cell pellicle spreading on the solid surface
or liquid media (Murray, 1963). Each cell is usually linked to the near cells
by a remarkable proteic surface structure or S layer (Murray, 1963 ; Austin and
Murray, 1990). Murray (1984) and Khun and Starr (1965) reported the presence of
refractile PHB inclusions when cells grow in presence of sodium acetate.
Several
isolation techniques have been tested; among them, the most reliable was the
direct spreading of sludge samples onto agar plates. Sequential steps onto agar
plates allowed culture isolation.
The solid medium
was modified GTA, kept at 25 °C in aerobic conditions. Once Lampropedia colonies were isolated from
the rest of the sludge population, several cultural enrichments on a liquid
medium containing 2 g·L-1 agar were tested. In this liquid medium, Lampropedia cells are mainly growing on
the liquid surface.
The pure culture
of Lampropedia were almost daily
renewed in new agar plates or in test tubes; the latter was more stable than
plates, since this kind of environment is slightly damp, as Lampropedia requires (Murray, 1963). The
properties of Lampropedia hyalina as
originaly described by Puttlitz and Seeley (1968) correspond to the used
strain.
Characterisation
tests, a group of those enumerated in Bergey’s manual (Tab.1), were carried out
in order to confirm the visual identification of Lampropedia;. According to test results, the used strain
corresponds to Lampropedia. The
species attribution, however, is not clearly defined.
Tab. 1. Identification tests for Lampropedia spp.
|
Characteristic |
reaction
or result obtained |
reaction
or result for Lampropedia spp.
(Bergey’s) |
|
Gram
staining |
– |
– |
|
Neisser
staining |
+ |
not
reported |
|
Growth
under anaerobic conditions |
– |
– |
|
Intracellular
PHB formed |
+ |
+ |
|
Catalase
test |
+ |
+ |
|
Nitrate
reduction |
– |
– |
|
Motility |
– |
– |
|
Shape
characteristic |
cocci
in sheet |
cocci
in sheet |
|
Cell
arranged in square tablets of 16-64 cells |
+ |
+ |
|
Final pH
culture media |
8.3-8.5 |
8.4-8.6 |
To check the
presence of PHB granules in the cells (Kuhn and Starr, 1965), Sudan Black
B and the more specific Blue Nile
A stains were used for microscopic observations. PHB was also measured by GC
analysis.
|
|
|
|
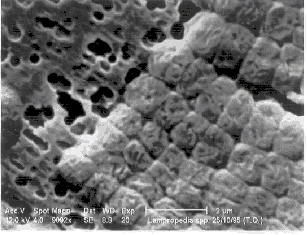
Fig. 1. Lampropedia spp. in pure culture (Sudan Black stain, white bar is
10 µm) |
Fig. 2. Lampropedia spp. (scanning electron microscope, bar corresponds to 2 µm) |
Growth
Growth tests
have been carried out in aerobic conditions on enriched (mod. GTA) and minimal
media (ADM), to calculate stoichiometric and kinetic parameters.
Trends of
substrate (sodium acetate) and biomass (calculated from VSS, not including the
weight of PHB) during 9 growth tests showed a decrease of substrate
concentration with correspondent biomass growth. In some tests, a slight
biomass growth after substrate consumption, probably due to degradation of
stored PHB, was observed. The same trend was observed with different initial
substrate concentration; however, growth rates varied depending on
substrate/biomass ratios.
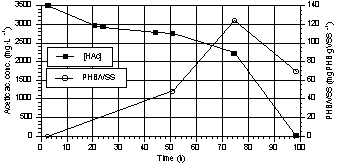
PHB was stored
also during aerobic cell growth, particularly with high substrate/biomass
ratios (Fig. 3). This phenomenon has been already described for other
microorganisms in unbalanced growth conditions (Dawes & Senior 1973).
PHB ranged from
3% to 12% w/w, with initial acetic acid concentrations ranging from 450 to 3500
mg·L-1 and initial acetic acid/VSS ratios of 3.5 and of 8.8
gHAc·gVSS-1 respectively. PHB/Acetate conversion factors were 6.5%
and 16% at the acetic acid
concentrations reported above.
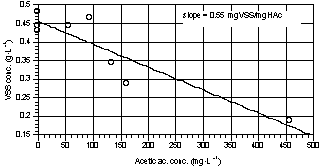
Fig. 4 shows the
VSS increase as a function of the removed substrate. The curve slope represents
the average biomass yield (0.55 gVSS·gHAc-1). The observed
COD:Nitrogen ratio consumed for growth was in average 100:2.
|
|
|
|
Fig. 3. Trend of PHB/VSS and acetic
acid during a growth test in aerobic conditions. |
Fig. 4. VSS trend as a function of
acetic acid depletion (curve slope represents the average yield). |
|
|
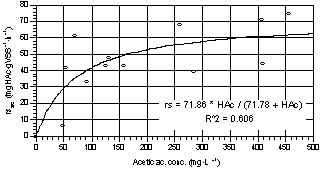
Fig. 5. Acetic acid specific removal
rate during growth as a function of acetic acid concentration (averaged
values of three parallel tests). |
On enriched
medium, the specific growth rate (µobs) was quite stable in the
substrate concentration range from 100 to 550 mg·L-1 of acetic acid,
with an average value of 0.045 h-1. Similar results were obtained on
minimal medium.
The specific
substrate removal rate (rsac) followed a Michaelis-Menten curve, as
reported in Fig. 5. From data interpolation, the estimated maximum rate was
71.9 mgHAc·gVSS-1·h-1 and the semisaturation constant
71.8 mgHAc·L-1.
Tests were
carried out to evaluate the capability of Lampropedia
spp to grow with nitrate as the final electron acceptor instead of oxygen:
no growth was detected.
Substrate uptake and PHB storage under anaerobic conditions
To evaluate
stoichiometric and kinetic parameters in anaerobic conditions, tests without
any inorganic electron acceptor and with sodium acetate as the sole substrate
were carried out.
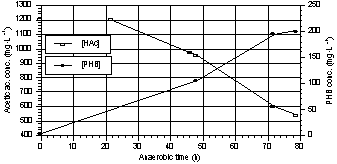
From Fig. 6 it
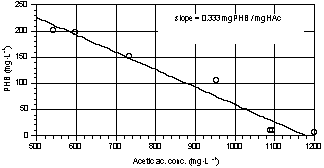
can be seen that acetate uptake is strictly related to PHB storage. The average
PHB yield, calculated as the slope of the curve in Fig. 7, was about 0.33
mgPHB·mgHAc-1; this value is lower than the one reported by Comeau (1986) and by
Smolders et al. (1994) for mixed
cultures, but it is quite similar to those reported by Fukase et al. (1982) and by Arun et al. (1988) in pure culture. This discrepancy can
partially depend on uncomplete extraction of PHB and on the fact that the
analytical method allowed only the estimation of PHB and not of other PHA. The
chromatograms showed qualitatively the presence of other methyl-volatile fatty
acids.
PHB was stored
into the cells in considerable amounts, about 31% of VSS dry weight.
The specific PHB
accumulation rate was strongly dependent on initial acetic acid concentration,
with higher values obtained at higher substrate concentration. The maximum rate
obtained was 17 mgPHB·gVSS-1·h-1. In accordance to this, specific
acetic acid uptake rate (31-57 mgHAc·gVSS-1·h-1) grew as a
function of initial acetic acid concentration.
|
|
|
|
Fig. 6. Trends of acetic acid and PHB
during a PHB storage test. |
Fig. 7. PHB vs acetic acid
concentration (the slope represents the average yield). |
Phosphorus release and uptake
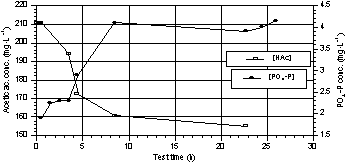
Tests on
phosphorus release under anaerobic conditions were carried out using acetic
acid as substrate. The rate of phosphorus release and acetic acid uptake was
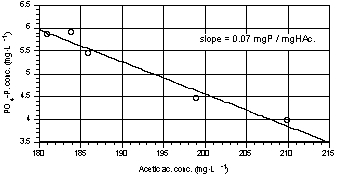
always higher at the beginning of the test (Fig. 8). Fig. 9 shows the
relationship between released orthophosphate and acetic acid uptaken in one
representative test; the slope represents the average stoichiometric ratio
obtained. The ratios between released orthophosphate and acetic acid removed
ranged in the various tests from 0.02 to 0.11 mgPrel·mgHAc-1rem,
with biomass concentration ranging from 0.2 to 0.64 gVSS·L-1. The
average value was 0.044 mgPrel·mgHAcrem-1.
These low values are comparable to those reported for Acinetobacter calcoaceticus by Ohtake et al. (1985) and for an isolated Gram + coccus by Ubukata and
Takii (1994), but lower than data obtained from poly-P activated sludge mixed
cultures. In average, the amount of P-PO4 released per gram of
biomass during the anaerobic phase was around 4 mg, in accordance to values of
2.5-4 mg reported for Acinetobacter
calcoaceticus by Ohtake et al.
(1985).
Biomass
phosphorus fractionation puts in evidence the presence of a significant amount
of polyphosphates. The cell culture showed a relevant difference in
polyphosphate content (5 mgP·gVSS-1) between the end of the anaerobic
phase and the end of the aerobic one (Tab. 2). It is possible to observe that poly-P
content drastically increases during the anaerobic/aerobic tests. These figures
confirm the previous values on phosphorus release reported above, and are also
similar to the values reported by Ohtake et
al. (1985).
Tab. 2. Phosphorus fractionation in Lampropedia cells exposed to alternate
aerobic/anaerobic conditions, compared to cells before alternate exposure.
|
phase |
polyphosphate
phosphorus (mgP·gVSS-1) |
other
cellular phosphorus (mgP·gVSS-1) |
|
end
of anaerobic |
2.42 |
11.42 |
|
end
of aerobic |
7.42 |
22.56 |
|
difference |
5 |
11.14 |
|
not
exposed cells |
1.6 |
11.4 |
In anaerobic
conditions, the specific PO4-P release rate ranged from 1.7 to 3.6
mgP-PO4·gVSS-1·h-1, with a pH near neutrality
(pH = 7.5). After about 5 hours, with only slight differences among different
tests, the rate slows down to values below 0.1 mgPO4-P·gVSS-1·h-1.
During all tests, the specific rate of PO4-P release did not show
statistically significant correlation with acetic acid and phosphorus
concentrations in the media, even if the highest values were obtained with the
highest substrate concentration and the lowest PO4-P concentration
at the beginning of each test.
On the contrary,
the specific acetic acid removal rate showed a significant correlation to substrate
concentration. The highest value obtained was 70 mgHAc·gVSS-1·h-1.
Phosphorus
uptake under aerobic condition showed a specific rate from 0.25 to 0.54 mgPO4-P·gVSS-1·h-1. This leads to an accumulation of
phosphorus in the biomass up to 3.5 % of VSS.
|
|
|
|
Fig. 8. Trend of acetic acid and
phosphorus during one of the P-release tests. |
Fig. 9. Phosphorus release vs acetic
acid uptake during one of the P-release tests. |
Conclusions
Enhanced
biological phosphate removal is extensively reported in literature on mixed
culture, but very few works have been successfully performed in pure culture,
and most of them do not report complete data on the whole uptake/release
mechanism of acetate and phosphorus.
In the present
work, a bacterium belonging to the genus Lampropedia
isolated from EBPR activated sludge has been studied for its capacity of
performing enhanced biological phosphate removal. Acetate uptake, PHB formation
and phosphate release in anaerobic conditions as well as phosphate uptake and
PHB consumption in aerobic conditions have been recorded. Stoichiometric and
kinetic values were usually lower than those obtainable in mixed culture, but
cell phosphorus fractionation analyses demonstrated a cyclic increase and
decrease of the poly-P fraction from aerobic to anaerobic conditions. All these
evidences allow to conclude that Lampropedia
spp. can be classified as a poly-P accumulating microorganism.
The easiness of
its visual recognition and its isolation in pure culture make Lampropedia spp. a candidate bacterium
for future pure culture experiments. Its study might contribute to a better
comprehension of the biochemical mechanism involved in enhanced biological
phosphate removal process.
Acknowledgements
Authors wish to
thank B. Biavati for their precious suggestions, A. Tarozzi, S. Gemelli and the
personnel and the director of Montecatini Environmental Research Center for
their collaboration to this research.
References
American Public Health Association (1989)
Standard Methods for examination of water and wastewater. 17°Ed., Washington
D.C.
Arun V., Mino T. and Matsuo T. (1988)
Biological mechanism of acetate uptake mediated by carbohydrate consumption in
excess phosphorus removal systems Water
Res. vol 22, No 5, 565-570.
Austin J. W. and R. G. E. Murray (1990)
Isolation and In Vitro Assembly of the Components of the Outer S Layer of Lampropedia hyalina.. Journal of Bacteriology 172,
7, 3681-3687
Bayly R. C., May J. W., Duncan A.,
Vasiliadis G., and Schembri M., (1990) Physiology of polyphosphate accumulating
Acinetobacter strain. Biological Nutrient Removal Conference,
Bendingo, Vittoria , Australia, 107–115
Bond P. L., Hugenholtz P., Keller J.,
Blackall L. (1995) Bacterial community structure of phosphate–removing and
non–phosphate–removing activated sludges from sequencing batch reactors. Appl.
Environ. Microbiol..,
61, 5, 1910–1916
Braunegg G. B., Sonnleitner and R. M.
Lafferty (1978) A rapid gas chromatographic method for determination of poli–ßhydroxybutiryc
acid in microbial biomass. Bior. Technol.
6, 29-37
Brodisch K E.U. and Joyner S. J. (1983)
The role of microorganism other than Acinetobacter
in biological sludge process. Water Sci.
Technol.. 15, 117-125
Comeau Y., Hall K. J., Hancock R.E.W. and
Oldham W.K. (1986) Biochemical model for enhanced biological phosphorus
removal. Water Res. 20, 12, 1511-1521
Dawes E. A. & Senior P.J. (1973) The
role and regulation of energy reserve polymers in microorganisms; Advanced in Microbial Physiology 10,
135–266
De Haas D. H. (1989) Fractionation of
bioaccumulation phosphorus compounds in activated sludge. Wat. Sci. Tech.. Vol. 21
Brighton, pp 1721-1725
Deinema M. H., Habets L. H. A., Scholten
J., Turkstra E. e Webers H. A. A. M. (1980) The accumulation of phosphate in Acinetobacter spp.. FEMS Microbiol. Lett. 9,
275-279
Deinema M. H., van Loosdrecht M. and
Scholten A. (1985) Some physiological charateristics of Acinetobacter spp. accumulating large amounts of phosphate. Wat. Sci. Technology. 17, 119-125
Florentz M. and Hartemann P. (1984)
Screening for phosphate accumulating bacteria isolated from activated sludge. Env. Tech. Letters . 5, 457-463.
Fuhs G.W. and Chen M. (1975)
Microbiological basis of phosphate removal in the activated sludge process for
the traetment of wastewater. Microbial
Ecology. 2, 119-138
Fukase T., Shibata M. and Miyaji Y. (1982)
Studies on the mechanism of biological phosphorus removal. Translate by C. J.
Mardon. Chem. Technol. Jap. J. Wat. Pollut. Res. 5, 309-317
Gerhardt P., Murray R.G.E., Wood W. A. e
Krieg N. R., (1994) Methods for General and Molecular Bacterioloy. American
Society for Microbiology, Washington D. C. pp. 529-530
Groenestijn et al. (1988)
Role of cations in accumulation and release of phosphate by Acinetobacter strain 210 A. Appl. Environ. Microbiol. 54, 2894-2901
Heymann J.B., Eagle L.M., Greben H.A. and
Potgieter D.J. (1989) The isolation and characterization of volutin granules as
subcellular components involved in biological phosphorous removal. Water Science Tech. 21, 397-408
Jenkins D., Richard M. G. and Daigger G. T.
(1989) Manual on the Causes and Control of Activated Sludge Bulking and
Foaming. Water Research Commission
Kuhn D.A. and M. P. Starr (1965) Clonal
Morphogenesis of Lampropedia hyalina..
Archiv. fur Mikrobiologie. 52, 360-375
Lötter L. H. and Murphy M. (1985) The
identification of heterotrophic bacteria in an activated sludge plant with
particular reference to polyphosphate accumulation. Water SA.. 11, 179–184.
Mino T., Arun V., Tsuzuki Y. and Matsuo T.
(1987) Effect of phosphorus accumulation on acetate uptake in biological
phosphorus removal process. Advanced in water pollution control: Biological
phosphate removal from wasterwater, Ramadori (ED) Pergamon Press, Oxford, 27-38
Mino T., Satoh H. e Matsuo T. (1994)
Metabolism of different bacterial populations in enhanced biological phosphate
removal process. Water Sci. Tech. 29,7, 67–70
Murray R.G.E. (1963) Role of superficial
structures in the characteristic morphology of Lampropedia hyalina.. Canadian
Journal of Microbiology. 9,
593-601
Murray R.G.E. (1984) Genus Lampropedia Schroeter 1886. Bergey’s
Manual of systematic bacteriology. Krieg and Halt ed.. The Williams and Wilkins Co.. 1, 402-406
Nakamura K., Hiraishi A., Yoshimi Y.,
Kawaharasaki M., Masuda K. and Kamagata Y. (1995) Microlunatus phosphorovorus gen. nov., sp. nov., a new
gram–positive polyphosphate–accumulating bacterium isolated from activated
sludge. International Journal of Systematic Bacteriology, 45, 1, 17–22
Nakamura K., Masuda K. and Mikami E.
(1991) Isolation of a new type of polyphosphate accumulating bacterium and its
phosphate removal characteristics. Journ.
of Ferment. and Bioengineering. 71,
4, 258-260
Ohtake H., Takahashi K., Tsukui Y. and
Kiyoshi T. (1985) Uptake and release of phosphate by a pure culture of Acinetobacter calcoaceticus.. Water Res.. 19, 12, 1587-1590
Ostle A.G.and Hot J.G. (1982) Nile blue A
as fluorescent stain for poly–ß–hydroxybutyrate. Appl Environ Microbiol.. 44, 238-241
Pasquinelli F.(1981)
Diagnostica e tecniche di laboratorio. Rosini ed. 2, 168-169.
Pringsheim E. G. (1955) Lampropedia hyalina Schroeter 1886 and
Vannielia aggregata n.g.,n.sp., with Remarks on Natural and on Organized
Colonies in Bacteria. J.Gen. Microbiol.. 13, 285-291
Pringsheim E. G. (1980) Type strain. Int. J. Syst. Bact.. 30, 314.
Puttlitz D. and Seeley H. V., (1968)
Phyciology and nutrition of Lampropedia
hyalina.. Journal of Bacteriology,
96, 4, 931–938.
Schroeter F.,
(1886) Bacterien in Cohn’s Kryptogamenflora
von Schlesien 3, 151
Smolders G. J. F., van der Meij J., van
Loosdrecht M. C. M. and Heijnen J.
J.(1994) Model of anaerobic metabolism of the biological phosphorus removal
process; stoichiometry and pH influence. Biotech. & Bioeng. 42, 461, 470
Standridge I. H. (1981) Poor Sludge
Settleability Caused by the Bacterium Lampropedia. Water Eng. & MGMT.. 128,
1, 41–43.
Tandoi V., Beccari M.,
Ramadori P. and Sebastiani Annichiarico L. (1987) Acinetobacter spp. growth in alternate anaerobic–aerobic
conditions. Advances
in Water Pollution Control, Biological Phosphate
Removal from Wasterwater. Ramadori R. Edit., Pergamon Press, Oxford, 305-308
Tandoi V., Blackall L.,
Caravaglio N. and Duranti F. (1990) Isolamento ed identificazione di specie
batteriche fosforo accumulanti e di attinomiceti producenti schiume. Istituto di Ricerca sulle Acque, Rapporti
Tecnici.. 131.
Ubukata Y. e Takii S., (1994) Induction
method of excess phosphate accumulation for phosphate removing bacteria
isolated from anaerobic/aerobic activated sludge. Water Sci. Techn.. 30, 6, 221-227.
Wagner M.,
Erhart R., Manz W., Amann R., Lemmer H., Wedi D., and Schleifer KH. (1994) Development of an rRNA–target oligonucleotide probe specific
for the genus Acinetobacter and its
application for situ monitoring in activated sludge. Appl.
Environ. Microbiol..60, 3; 792–800
Wentzel, M. C., Lötter, L. H., Loewenthal,
R. E. and Marais GyR. (1986) Metabolic behavior of Acinetobacter spp. in enhanced biological phosphorus removal–a
biochemical model. Water SA.. 12, 4, 209–224